 Penile erection is the most obvious feature of the male body's response to sexual excitement. It is a complex neurovascular response, influenced by cognitive inputs and facilitated by testosterone. Other features of that response include increases in skin temperature, blood pressure, heart and breathing rates, facial and bodily flushing, dilation of the pupils, and nipple erection. There are also changes in skin's sensitivity to touch. These changes are similar in both men and women.
Penile erection is the most obvious feature of the male body's response to sexual excitement. It is a complex neurovascular response, influenced by cognitive inputs and facilitated by testosterone. Other features of that response include increases in skin temperature, blood pressure, heart and breathing rates, facial and bodily flushing, dilation of the pupils, and nipple erection. There are also changes in skin's sensitivity to touch. These changes are similar in both men and women.Erection response to sexual interest is the result of interplay between tactile, visual, auditory, and olfactory signals, combined with cognitive inputs, such as fantasy and memory (Figure 1). These stimuli may be erectogenic or erectolytic, pleasant or unpleasant, and are integrated in specific nuclei within the mid-brain. This balance between stimuli may result in pro-erectile signaling transmitted via the spinal cord, pelvic nerves and cavernous nerves running either side of the prostate gland, before finally terminating around the vascular smooth muscle of the corpora cavernosa.
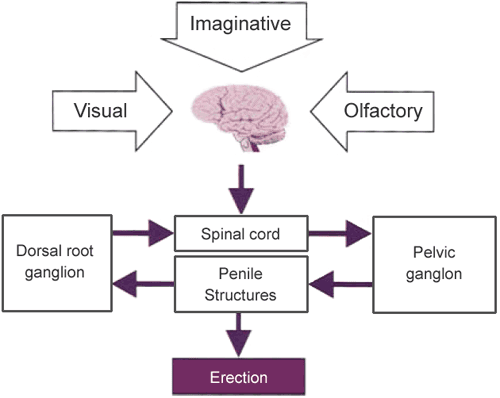
Figure 1: Penile erection is basically a spinal reflex controlled supraspinally, where erectile stimuli can he generated in response to visual, olfactory, and imaginative stimuli.
Erections can also occur in the absence of sexual stimulation. Most men will get spontaneous erections in their sleep, unrelated to sexual thoughts or dreams. Contrary to popular belief, they are not caused by having a full bladder, but probably by unconscious and involuntary changes in the electrical activity of the brain. Men can also get 'reflex' erections, due to activity of sensory-motor linkages within the spinal cord. These are common in men with multiple sclerosis and some types of spinal cord injury.
Erection is a complex series of integrated vascular events culminating in the accumulation of blood under pressure and end-organ rigidity. Fundamentally involved in the intrapenile response are the paired corpus cavernosa that contain the erectile components and are surrounded by a thick fibro-elastic sheath, the tunica albuginea. The erectile tissue comprises multiple interconnecting sinusoidal spaces or lacunae surrounded by trabeculae of smooth muscle (Figure 2).

Figure 2: Cross-section of the penis.
During erection there is a considerable (up to eightfold) increase in the effective intrapenile blood volume, with corresponding expansion of the trabecular walls and lacunar spaces (Figure 3).
Compression of the plexus of subtunical venules follows, reducing venous outflow. This phenomenon, often referred to as the veno-occlusive mechanism, produces an increase in penile volume (tumescence) and rigidity.

Figure 3: The veno-occlusive mechanism of penile erection.
Detumescence occurs through the reversal of this process following contraction of penile smooth muscle. The activation of sympathetic constrictor fibers causes an increase in the tone of the helicine arteries and the trabeculae. Arterial inflow is reduced and the lacunar space collapses. Decompression of the subtunical venules follows, resulting in increased venous outflow. The penis is returned to the flaccid state.
The overall tone of cavernosal smooth muscle represents an integrated response to many different pathways and systems, the details of which are incompletely understood.
Peripherally, the local balance between contractant and relaxant factors controls the degree of contraction of the smooth muscle and determines the functional status of the penis.
As elsewhere within the autonomic nervous system, sympathetic noradrenergic fibers and parasympathetic cholinergic fibers innervate the cavernosal tissue. In addition, there is innervation from non-adrenergic, non-cholinergic (NANC) nerves.
Within the NANC system a neuromodulator, nitric oxide, is released by nerve endings and endothelial cells, and acts postjunctionally to activate an intracellular enzyme cascade, culminating in erection.
Nitric oxide increases the production of cyclic guanosine monophosphate (cGMP) that decreases intracellular calcium, causing smooth muscle relaxation and penile engorgement (Figure 4). In turn, cGMP is broken down by phosphodiesterases (PDE5). Inhibition of this enzyme forms the basis of the phosphodiesterase inhibitor story.
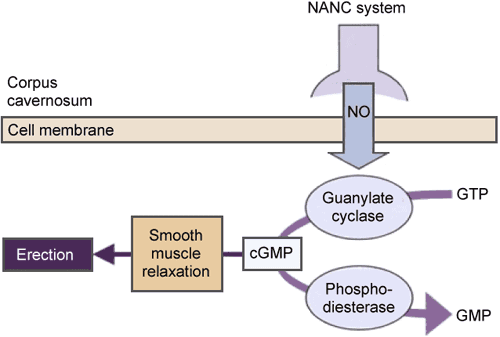
Figure 4: The role of nitric oxide (NO), cyclic guanosine monophosphate (cQMP), and phosphodiesterase type 5 (PDE5) in penile erection.
The central nervous system (CNS) is fundamentally involved in the maintenance of normal erectile and sexual function as it initiates signaling to the penis via the autonomic nervous system and integrates the overall response. An action within the CNS could therefore represent an attractive option as a site of action of an erectogenic drug.
Apomorphine SL was the first example of a centrally active drug approved for use in ED patients; other agents, with a range of target receptors, are being studied. Apomorphine is known to act on dopamine receptors in the brain (Figure 5). A signal is initiated that then travels within the autonomic nervous system to nerves in the pelvic area, dilating the cavernosal vasculature and causing an erection.

Figure 5: Schematic representation of the involvement of dopaminergic pathways in the control of erectile function in the rat.
As normal erectile function depends on the delicate balance between vasorelaxation and vasoconstriction of the corporal smooth muscle, disruption of this equilibrium can result in ED. If this critical level of smooth muscle relaxation is not achieved, there will be incomplete resistance to the venous outflow of blood from the corpora and full penile tumescence will be compromised. This is described as veno-occlusive dysfunction and can result from deficiency of the various systems that support the normal integrated response for penile erection.
Vascular factors: Probably the most frequent causes of organic ED are endothelial dysfunction and disturbance of smooth muscle responsiveness within the corporal tissue of the penis.
Decreased arterial flow and perfusion pressure to the lacunar spaces can result from atherosclerosis, or traumatic arterial occlusive disease, of the hypogastric-cavernous arterial bed and may also be a contributory factor.
Excessive outflow through the subtunical venules may result in incomplete tumescence despite sufficient arterial inflow. This can be caused by insufficient relaxation of the trabecular smooth muscle, which may occur in anxious patients with excessive adrenergic-constrictor tone, through damage to the parasympathetic dilator nerves, or by corporal smooth muscle dysfunction.
Neurological factors: Disorders affecting the sacral spinal cord or the peripheral efferent autonomic fibers to the penis can result in incomplete relaxation of the trabecular smooth muscle. Also, disruption of the somatic fibers from the penis that transmit sensory stimuli to the thalamus and sensory cortex (via the pudendal nerve) may also result in ED.
Such neurogenic ED can arise from spinal cord injury, multiple sclerosis, peripheral neuropathies (secondary to diabetes), alcoholism, surgical procedures such as radical prostatectomy, or pelvic radiation therapy.
Endocrinological factors: Androgens are necessary for normal sexual development but also influence sexual motivation and behavior. Androgens have been shown to influence the activity of nitric oxide synthase (NOS) and smooth muscle relaxation in the corpus cavernosum. Low levels of bioavailable testosterone may result from a wide range of causes, including changes in the sensitivity of the hypothalamic—pituitary—gonadal axis due to aging, primary hypogonadism, hyperprolactinemia, and the use of leutinizing hormone-releasing hormone (LHRH) agonists.
Diabetes is the most common endocrine abnormality associated as a risk factor for ED. ED may eventually develop in 60% of men with diabetes mellitus. The main causes of the associated ED are thought to arise from the vasculogenic and neurological sequelae of the diabetes. In particular, endothelial and smooth muscle dysfunction and neurological damage to C fibers have been implicated. As many as 40% of diabetic men are also hypogonadal.
ED is also associated with hyperthyroidism.
Psychogenic factors: The brain is the most important source of pro-erectile signaling in response to sexual stimulation. Unpleasant tactile, visual, auditory, and olfactory stimuli will tend to inhibit erection. Unpleasant sexual fantasies, perhaps envisaging embarrassment and rejection due to loss of erection with a partner, and memory of poor past sexual experience or relationship dysfunction, will have a similar effect. If these unpleasant, erectolytic stimuli predominate, there may be inadequate central pro-erectile signaling to provide the degree of sustained cavernosal smooth muscle relaxation required for erection.
Penile factors: Peyronie's disease is associated with ED, although it may coexist with other causal factors. In a recent study, nearly a third of men with untreated Peyronie's disease were found to have ED. Changes in the integrity of the fibroelastic components of the trabeculae may result in reduced compression of the subtunical venules. This may be the result of aging, increased cross-linkage of collagen fibers (induced by non-enzymatic glycosylation and hypoxia), altered collagen synthesis associated with hypercholesterolemia, or by trauma to the penis.
by Culley C. Carson and John D. Dean
No comments:
Post a Comment